Key Takeaways
- IGF-1 (Insulin-like Growth Factor 1) plays a crucial role in vascular health and tissue repair, making it significant for erectile function
- Clinical studies have established correlations between low IGF-1 levels and erectile dysfunction in men
- IGF-1 supports erectile function through multiple mechanisms: enhancing nitric oxide production, maintaining smooth muscle integrity, and supporting nerve health
- Age, metabolic conditions, and lifestyle factors can all influence IGF-1 levels, potentially impacting erectile capacity
- Emerging therapeutic approaches targeting IGF-1 show promise for treating erectile dysfunction, particularly in cases resistant to conventional treatments
Introduction to IGF-1 and Erectile Function
Insulin-like Growth Factor 1 (IGF-1) is a peptide hormone structurally similar to insulin, primarily produced by the liver in response to growth hormone stimulation. This powerful anabolic mediator plays essential roles throughout the body, including cell growth, tissue repair, and importantly for our discussion, vascular homeostasis. As a circulating hormone, IGF-1 has endocrine effects on distant tissues, including the male reproductive system and its vascular components.
Erectile function depends on a complex interplay of vascular, neural, and endocrine factors working in harmony. At its physiological core, an erection requires coordinated vasodilation of penile arteries, relaxation of smooth muscle in the corpus cavernosum, and activation of veno-occlusive mechanisms to maintain blood trapping. This process relies heavily on nitric oxide (NO) release from endothelial cells and nerve terminals, which triggers smooth muscle relaxation through cyclic guanosine monophosphate (cGMP) pathways.
The connection between IGF-1 and erectile capacity stems primarily from its profound effects on vascular endothelial health and neurovascular integrity. IGF-1 has been shown to enhance endothelial nitric oxide synthase (eNOS) activity, promote vascular regeneration, and protect against oxidative stress—all critical factors in maintaining erectile capacity. Furthermore, IGF-1 supports peripheral nerve health and regeneration, which is essential for the neurological aspects of erectile function.
Erectile dysfunction (ED) affects approximately 30 million men in the United States alone, with prevalence increasing significantly with age. According to the Massachusetts Male Aging Study, about 40% of men experience some degree of ED by age 40, rising to nearly 70% by age 70 (Feldman et al., 1994). Despite the availability of PDE5 inhibitors like sildenafil (Viagra), many men either don't respond adequately to these treatments or experience diminishing effects over time, highlighting substantial unmet therapeutic needs in this field.
In the following sections, we will examine the compelling clinical evidence linking IGF-1 levels to erectile function, explore the molecular mechanisms through which IGF-1 influences erectile physiology, and discuss how this knowledge may shape future therapeutic approaches for men suffering from erectile dysfunction.
Clinical Evidence Linking IGF-1 Levels to Erectile Dysfunction
Substantial clinical research has established correlations between circulating IGF-1 levels and erectile function, providing a foundation for understanding this hormone's role in male sexual health. Multiple observational studies have demonstrated that men with erectile dysfunction often present with lower serum IGF-1 concentrations compared to age-matched controls with normal erectile function.
One particularly significant investigation by Açıkgöz and colleagues (2016) examined 80 patients with varying degrees of erectile dysfunction alongside 40 healthy controls. The researchers found that mean serum IGF-1 levels were significantly lower in men with ED (142.5 ± 36.1 ng/mL) compared to those without ED (194.5 ± 34.6 ng/mL, p < 0.001). More tellingly, IGF-1 levels correlated negatively with erectile dysfunction severity as measured by the International Index of Erectile Function (IIEF) questionnaire—a validated tool for assessing erectile function (Açıkgöz et al., 2016).
A complementary study by Shindel and colleagues (2016) investigated age-specific correlations between IGF-1 and sexual function in 1,556 men aged 30-79 years. After adjusting for multiple variables, including age, body mass index, and comorbidities, they found that lower IGF-1 levels were independently associated with increased odds of experiencing erectile dysfunction (OR = 1.3, 95% CI 1.1-1.5) (Shindel et al., 2016).
These findings gain further support from the work of Schulster and colleagues (2018), who conducted a case-control study involving 129 men with ED and 122 controls. They observed that men in the lowest quartile of IGF-1 levels had a 2.7-fold increased risk of having moderate to severe erectile dysfunction compared to those in the highest quartile, even after adjusting for age, testosterone levels, and metabolic factors (Schulster et al., 2018).
The potential utility of IGF-1 as a biomarker for ED has gained attention based on these correlations. When evaluated for diagnostic accuracy in a study by Zhang et al. (2020), serum IGF-1 showed a sensitivity of 78% and specificity of 71% for predicting moderate to severe ED, with an area under the ROC curve of 0.76 (Zhang et al., 2020). While these values suggest potential clinical utility, they also indicate that IGF-1 alone is not sufficient as a standalone diagnostic marker, but rather should be considered within a comprehensive assessment.
It's crucial to acknowledge that these studies establish association rather than causation. Many of these investigations are cross-sectional in design, providing a snapshot of the relationship between IGF-1 and erectile function at a specific point in time. However, the consistency of findings across different populations and methodologies strengthens the evidence for a meaningful biological connection between IGF-1 and erectile capacity.
Longitudinal studies examining whether declining IGF-1 levels precede the development of ED would provide stronger evidence for a causal relationship. Nevertheless, the robust associations observed across multiple clinical investigations, coupled with the mechanistic plausibility that will be explored in the next section, provide compelling support for IGF-1's role in erectile physiology.
Molecular and Cellular Mechanisms: How IGF-1 Influences Erectile Function
The clinical associations between IGF-1 levels and erectile function are supported by extensive molecular evidence elucidating how this growth factor influences the key physiological pathways governing erection. IGF-1 exerts its effects through multiple mechanisms that collectively enhance vascular function, protect smooth muscle integrity, and support neuronal health in penile tissues.
Endothelial Function and Nitric Oxide Signaling
At the vascular level, IGF-1 significantly enhances endothelial function, which is paramount for erectile capacity. Research has demonstrated that IGF-1 upregulates endothelial nitric oxide synthase (eNOS) expression and activation through the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway (Imrie et al., 2010). This upregulation leads to increased nitric oxide (NO) production—the principal mediator of penile smooth muscle relaxation and subsequent erection.
IGF-1 also protects endothelial cells from oxidative stress and inflammation, two processes that can impair NO bioavailability. By activating antioxidant defense mechanisms, including superoxide dismutase and glutathione peroxidase, IGF-1 helps maintain the redox balance necessary for optimal endothelial function (Csiszar et al., 2008). This protective effect is particularly relevant in conditions like diabetes and aging, where oxidative stress contributes significantly to endothelial dysfunction and subsequent ED.
Smooth Muscle Integrity and Function
Beyond its endothelial effects, IGF-1 plays a crucial role in maintaining the structural and functional integrity of corpus cavernosum smooth muscle. Research using both animal models and human tissue samples has shown that IGF-1 prevents smooth muscle atrophy and fibrosis—a common pathological finding in chronic ED (Jiang et al., 2020).
IGF-1 accomplishes this protection through multiple mechanisms, including:
- Inhibition of transforming growth factor-beta (TGF-β)-induced collagen production
- Reduction of reactive oxygen species that promote fibrotic changes
- Stimulation of smooth muscle cell proliferation to maintain tissue architecture
- Enhancement of cellular energy metabolism for optimal contractile function
NO-cGMP Signaling Pathway Enhancement
The NO-cGMP signaling cascade is the principal molecular pathway mediating penile erection. After NO is produced by endothelial cells or nitrergic neurons, it diffuses into adjacent smooth muscle cells, activating soluble guanylate cyclase (sGC) to produce cGMP from GTP. The resulting elevation in cGMP activates protein kinase G (PKG), which phosphorylates various targets leading to decreased intracellular calcium and smooth muscle relaxation.
IGF-1 enhances this pathway at multiple levels. Beyond increasing NO production as discussed above, IGF-1 has been shown to upregulate sGC expression and activity, amplifying the downstream effects of NO (Musicki et al., 2015). Additionally, IGF-1 may enhance the sensitivity of smooth muscle cells to NO by modulating phosphodiesterase (PDE) expression, the enzymes responsible for cGMP degradation.
Neuronal Support and Regeneration
The neuronal component of erectile function is equally critical and significantly influenced by IGF-1. Studies have demonstrated that IGF-1 promotes peripheral nerve regeneration following injury—a finding with particular relevance for post-surgical or diabetic ED, where neuropathy plays a central role (Sezen et al., 2001).
At the molecular level, IGF-1 enhances neuronal survival and axonal growth through activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and the mitogen-activated protein kinase (MAPK) pathway. Furthermore, IGF-1 increases the expression of neuronal nitric oxide synthase (nNOS) in cavernous nerves, thereby enhancing the neural contribution to NO production during sexual stimulation (Lin et al., 2006).
These molecular mechanisms collectively explain the clinical observations linking IGF-1 levels to erectile function. By simultaneously enhancing endothelial function, preserving smooth muscle integrity, amplifying NO-cGMP signaling, and supporting neuronal health, IGF-1 comprehensively addresses the physiological requirements for normal erectile function. The multifaceted nature of IGF-1's effects also explains why deficiencies in this growth factor can lead to erectile dysfunction through multiple parallel pathways.
Experimental Evidence from Animal Models
Animal models have provided invaluable insights into the causal relationship between IGF-1 and erectile function, allowing for controlled experimental manipulations not possible in human studies. These models have confirmed the mechanistic connections proposed based on clinical observations and molecular investigations.
IGF-1 Gene Therapy Studies
Some of the most compelling evidence comes from gene therapy studies in aging rats, where declining erectile function naturally parallels the human condition. Zhou and colleagues (2013) demonstrated that intracavernosal injection of adeno-associated viral vectors carrying the IGF-1 gene significantly improved erectile function in aged rats. Treated animals showed approximately 70% higher intracavernosal pressure (ICP)/mean arterial pressure (MAP) ratios compared to age-matched controls, approaching levels seen in young healthy animals (Zhou et al., 2013).
The functional improvements correlated with enhanced endothelial markers, including increased eNOS and phospho-eNOS expression in penile tissue, confirming the molecular mechanisms proposed earlier. Importantly, these benefits persisted for up to 6 months after a single treatment, suggesting durable therapeutic potential.
Direct Protein Administration Studies
Complementary studies using direct administration of recombinant IGF-1 protein have shown dose-dependent improvements in erectile function across various animal models. Pu and colleagues (2012) reported that intracavernosal injection of IGF-1 protein in diabetic rats significantly improved erectile responses within 30 minutes, with effects lasting approximately 24 hours. At the optimal dose (5 μg), IGF-1 increased the ICP/MAP ratio by 62% compared to untreated diabetic controls (Pu et al., 2012).
Histological examinations revealed that IGF-1 treatment preserved corpus cavernosum smooth muscle content and reduced fibrosis. Molecular analyses showed increased phosphorylation of eNOS and Akt, confirming activation of the key signaling pathways described in the previous section.
Efficacy in Specific ED Models
IGF-1 interventions have shown particular promise in models of neurogenic and diabetic erectile dysfunction—two conditions that often respond poorly to conventional PDE5 inhibitor therapy.
In cavernous nerve injury models mimicking post-prostatectomy ED, Fandel and colleagues (2009) demonstrated that IGF-1 treatment accelerated nerve regeneration and preserved erectile function. Treated animals retained approximately 70% of normal erectile capacity compared to just 35% in untreated controls (Fandel et al., 2009).
Similarly, in streptozotocin-induced diabetic rat models, IGF-1 therapy improved both the magnitude and duration of erectile responses. Importantly, these benefits were observed even in animals with severe diabetes, suggesting potential application to advanced diabetic ED cases (Hinkel et al., 2011).
Limitations of Animal Models
While animal studies provide strong experimental support for IGF-1's role in erectile function, several limitations should be acknowledged:
- Species differences in penile anatomy and physiology may limit direct translation to humans
- Most studies use relatively young animals with acute disease models, whereas human ED often develops gradually over decades
- The delivery methods used (direct intracavernosal injection or gene therapy) present practical challenges for clinical application
- Few studies have examined potential systemic side effects of IGF-1 therapy over extended periods
Despite these limitations, the consistency of findings across different animal models, intervention types, and research groups strongly supports the causal relationship between IGF-1 and erectile function. The convergence of these experimental data with human clinical observations and molecular mechanisms provides a robust scientific foundation for considering IGF-1 as both a biomarker and therapeutic target for erectile dysfunction.
Factors Affecting IGF-1 Levels and Erectile Function
Multiple physiological, pathological, and lifestyle factors influence circulating IGF-1 levels and, consequently, may impact erectile function. Understanding these factors helps explain the variable presentation of erectile dysfunction among different individuals and suggests potential interventional approaches beyond direct IGF-1 administration.
Age-Related Decline
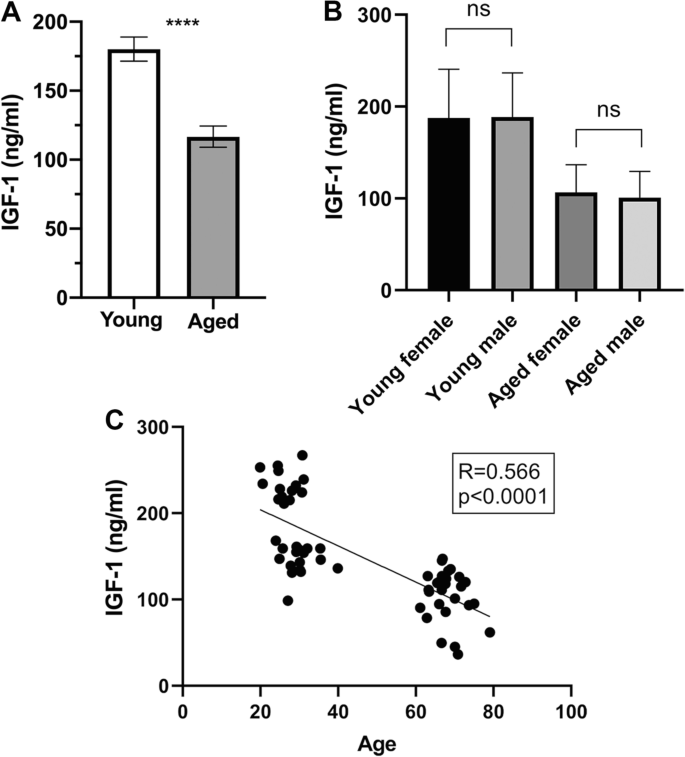
Perhaps the most consistent factor affecting IGF-1 is age. After reaching peak levels during puberty, IGF-1 concentrations decline gradually throughout adulthood, with most men experiencing a 30-50% reduction between ages 20 and 80 (Juul et al., 1997). This decline parallels the age-related increase in ED prevalence, which rises from approximately 40% at age 40 to nearly 70% by age 70.

Image Source: Toth, L., Czigler, A., Hegedus, E. et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. GeroScience 44, 2771–2783 (2022). https://doi.org/10.1007/s11357-022-00623-2
The age-dependent reduction in IGF-1 contributes to several pathophysiological changes in penile tissue, including:
- Decreased elasticity and compliance of vascular and cavernous tissues
- Reduced endothelial regenerative capacity, leading to progressive endothelial dysfunction
- Diminished smooth muscle content relative to connective tissue (fibrosis)
- Impaired neuronal plasticity and repair following minor injuries
These structural and functional changes create a biological environment conducive to erectile dysfunction, explaining in part why advancing age remains the strongest risk factor for ED.
Metabolic Disorders and Insulin Resistance
Metabolic conditions—particularly diabetes, obesity, and metabolic syndrome—significantly impact IGF-1 bioavailability and signaling. Interestingly, these conditions can affect IGF-1 in complex ways: total IGF-1 levels may be normal or even elevated, but free (bioactive) IGF-1 is often reduced due to alterations in IGF binding proteins (IGFBPs) (Friedrich et al., 2012).
More importantly, insulin resistance, the common denominator in these metabolic disorders, impairs IGF-1 signaling at the receptor and post-receptor levels. This "IGF-1 resistance" means that even normal circulating levels cannot exert their full biological effects on target tissues, including the penis.
The link between metabolic disorders and ED is well-established, with diabetic men experiencing ED rates up to three times higher than non-diabetic counterparts. While multiple mechanisms contribute to this association, impaired IGF-1 signaling represents an important pathophysiological pathway that helps explain why diabetic ED often proves resistant to conventional treatments.
Hormonal Regulation and Interactions
IGF-1 exists within a complex hormonal network, with growth hormone (GH) serving as its primary regulator. However, other hormones significantly influence IGF-1 production and activity, including:
- Testosterone: Enhances GH secretion and IGF-1 production; also upregulates IGF-1 receptors in target tissues
- Thyroid hormones: Stimulate IGF-1 synthesis and potentiate IGF-1 effects at the tissue level
- Cortisol: Chronically elevated levels inhibit IGF-1 production and signaling
- Insulin: Increases hepatic IGF-1 production but can reduce free IGF-1 bioavailability
These hormonal interactions help explain certain clinical observations, such as the frequent co-existence of hypogonadism and ED, or the erectile benefits sometimes observed with growth hormone replacement in deficient individuals.
Lifestyle and Environmental Factors
Numerous modifiable factors affect IGF-1 levels, creating opportunities for lifestyle-based interventions:
-
Nutrition: Protein intake significantly influences IGF-1 production, with both severe protein restriction and optimal protein consumption affecting levels. Additionally, micronutrients like zinc and magnesium serve as cofactors for IGF-1 synthesis and action.
-
Physical activity: Regular moderate-intensity exercise increases IGF-1 levels and improves IGF-1 sensitivity in target tissues. Conversely, excessive high-intensity exercise may temporarily suppress IGF-1 through elevated cortisol.
-
Sleep quality: Growth hormone secretion—and consequently IGF-1 production—occurs predominantly during deep sleep. Chronic sleep disruption or insufficiency reduces IGF-1 levels.
-
Chronic stress: Persistently elevated cortisol due to psychological stress inhibits the GH/IGF-1 axis. This connection may partially explain stress-related erectile difficulties.
The interplay between these various factors creates a complex physiological environment affecting IGF-1 status. Understanding these relationships helps explain individual variability in erectile function among men of similar ages and medical backgrounds. More importantly, it identifies potential therapeutic targets beyond direct IGF-1 administration, including lifestyle modifications and hormonal optimizations that may enhance endogenous IGF-1 production and signaling.
Therapeutic Potential of IGF-1 in Erectile Dysfunction
The established link between IGF-1 and erectile function has sparked significant interest in therapeutic applications targeting this pathway, particularly for men who respond poorly to conventional treatments like PDE5 inhibitors. Various approaches are being investigated, each with unique advantages, limitations, and developmental status.
Direct IGF-1 Administration
The most straightforward approach involves administering recombinant human IGF-1 (rhIGF-1) directly to penile tissue. In preclinical models, intracavernosal injection of rhIGF-1 has demonstrated rapid improvements in erectile function, with effects typically lasting 24-48 hours (Pu et al., 2012).
This approach offers several advantages:
- Targeted delivery to penile tissue, minimizing systemic exposure
- Rapid onset of action
- Potential efficacy in patients unresponsive to PDE5 inhibitors
However, significant challenges remain, including the need for repeated injections, potential discomfort associated with the delivery method, and limited data on long-term efficacy and safety in humans. Early-phase clinical trials are currently evaluating optimal dosing, administration frequency, and safety profiles.
Gene Therapy Approaches
IGF-1 gene therapy represents a more durable approach, potentially providing continuous local production of IGF-1 in penile tissue following a single administration. Preclinical studies using viral vectors (primarily adeno-associated viruses) to deliver the IGF-1 gene have demonstrated sustained improvements in erectile function lasting up to 6 months (Zhou et al., 2013).
The primary advantages of gene therapy include:
- Long-lasting effects from a single treatment
- Steady-state production of IGF-1 within the target tissue
- Potential for combination with other therapeutic genes (e.g., eNOS)
However, this approach faces considerable regulatory hurdles and safety concerns, including potential immune responses to viral vectors, theoretical risks of insertional mutagenesis, and challenges in controlling gene expression levels once delivered. Clinical translation will require extensive safety validation before progressing to efficacy trials.
Stem Cell Therapy Leveraging IGF-1
An emerging therapeutic strategy involves stem cell transplantation, capitalizing on the ability of certain stem cell populations to secrete IGF-1 and other growth factors. Mesenchymal stem cells (MSCs) and adipose-derived stem cells (ADSCs) have shown particular promise, with transplantation into corpus cavernosum tissue improving erectile function in models of aging, diabetes, and cavernous nerve injury (Chen et al., 2016).
The beneficial effects appear mediated partly through paracrine IGF-1 secretion, as confirmed by studies showing reduced efficacy when IGF-1 signaling is blocked. Some researchers have enhanced this approach by genetically modifying stem cells to overexpress IGF-1, creating "super-secretor" cell populations with greater therapeutic potential.
Advantages of stem cell approaches include:
- Multifactorial effects beyond IGF-1 (including angiogenic, anti-inflammatory, and anti-fibrotic effects)
- Potential for enduring benefits as cells integrate into host tissue
- Generally favorable safety profile in early clinical studies
Challenges include standardization of cell populations, optimizing cell survival following transplantation, and determining the most effective delivery protocols.
IGF-1 Sensitizers and Indirect Modulators
Rather than directly administering IGF-1, some therapeutic strategies aim to enhance endogenous IGF-1 production or signaling. These approaches include:
- Growth hormone secretagogues that stimulate pituitary GH release and subsequent hepatic IGF-1 production
- IGF binding protein (IGFBP) modulators that increase free (bioactive) IGF-1 levels
- Post-receptor signaling enhancers that amplify IGF-1's effects without increasing hormone levels
While these approaches may offer advantages in terms of systemic safety and ease of administration (potentially allowing oral delivery), they remain in early developmental stages with limited erectile-specific data.
Safety Considerations and Regulatory Outlook
Despite promising efficacy data, several safety concerns must be addressed before IGF-1-based therapies can advance to widespread clinical use:
- Fibrosis: Supraphysiological IGF-1 levels could potentially promote penile fibrosis over time
- Priapism: Enhanced NO signaling might theoretically increase priapism risk in susceptible individuals
- Carcinogenic potential: IGF-1's growth-promoting properties raise theoretical concerns about accelerating latent prostate cancer or other malignancies
- Systemic effects: Even with local administration, some systemic absorption occurs, potentially affecting glucose metabolism and other IGF-1-responsive systems
The regulatory pathway for these novel therapies will likely require comprehensive safety evaluations, especially for gene therapy approaches. Initial approvals may be limited to patients with severe, treatment-resistant ED or specific etiologies (e.g., post-prostatectomy or diabetic ED) where benefit-risk calculations are most favorable.
Summary of Scientific Proof and Clinical Implications
The scientific evidence supporting IGF-1's role in erectile function spans multiple investigational domains, creating a coherent picture that extends from molecular mechanisms to clinical observations. This multifaceted evidence base provides strong scientific proof for IGF-1's importance in penile physiology and its potential as a therapeutic target.
Clinical studies have consistently demonstrated associations between low IGF-1 levels and erectile dysfunction across diverse populations. These correlations remain significant even after adjusting for common confounding factors like age, comorbidities, and testosterone status, suggesting an independent relationship. The dose-response pattern observed in several studies—where decreasing IGF-1 quartiles correlate with increasing ED severity—further strengthens the evidence for a biologically meaningful connection.
At the molecular level, IGF-1 influences multiple pathways critical for erectile function. By enhancing endothelial nitric oxide production, preserving smooth muscle integrity, amplifying NO-cGMP signaling, and supporting neuronal health, IGF-1 comprehensively addresses the core physiological requirements for normal erections. These mechanisms provide biological plausibility for the clinical associations observed.
Experimental evidence from animal models offers the most direct causal support, demonstrating that IGF-1 administration—whether as protein, gene therapy, or through stem cell delivery—improves erectile function in various models of dysfunction. These improvements correlate with favorable changes in tissue structure and molecular signaling pathways, confirming the mechanistic hypotheses and suggesting therapeutic potential.
The convergence of evidence across these different domains—clinical, molecular, and experimental—creates a robust scientific foundation supporting IGF-1's critical role in erectile physiology and its potential as both a biomarker and therapeutic target for erectile dysfunction.
Nevertheless, important limitations and research needs remain:
- Large-scale, prospective clinical trials examining whether IGF-1 levels predict future ED development would strengthen the evidence for causality
- Optimization of delivery methods for IGF-1-based therapies remains necessary to balance efficacy, convenience, and safety
- Long-term safety data, particularly regarding fibrosis and theoretical cancer risks, are essential before widespread clinical application
- Identification of patient subgroups most likely to benefit from IGF-1-targeted approaches would enable more precise therapeutic strategies
Despite these ongoing challenges, the scientific foundation supporting IGF-1's role in erectile function is substantial and growing. For clinicians and patients dealing with erectile dysfunction, particularly treatment-resistant cases, this research opens promising new avenues for both diagnostic assessment and therapeutic intervention.
Frequently Asked Questions (FAQs)
Is low IGF-1 always indicative of erectile dysfunction?
No, low IGF-1 is not always indicative of erectile dysfunction. While statistical associations exist between lower IGF-1 levels and increased ED risk, many men with relatively low IGF-1 maintain normal erectile function. Erectile physiology depends on multiple factors beyond IGF-1, including vascular health, neurological integrity, hormonal balance, and psychological wellbeing. IGF-1 should be viewed as one contributor within this complex system rather than a definitive marker. Clinical studies suggest that IGF-1 levels in the lowest quartile for age increase ED risk approximately 2-3 fold, but many men with low-normal levels maintain satisfactory erectile function, particularly in the absence of other risk factors.
What is the difference between IGF-1 and growth hormone in erectile function?
Growth hormone (GH) and IGF-1 work in tandem as part of the somatotropic axis, but their roles in erectile function differ in important ways. GH is primarily a regulatory hormone produced by the pituitary gland that stimulates IGF-1 production in the liver and other tissues. While GH can directly influence some aspects of cellular metabolism, most of its growth-promoting and tissue-regenerative effects occur through IGF-1.
In erectile physiology, IGF-1 appears to play the more direct role by enhancing endothelial function, supporting smooth muscle maintenance, and promoting neuronal health in penile tissues. IGF-1 directly activates the PI3K/Akt pathway, which increases endothelial nitric oxide production—a critical mediator of erection. Growth hormone's effects on erectile function are largely indirect, occurring through its stimulation of IGF-1 production and release.
This distinction has therapeutic implications: IGF-1 administration provides more immediate effects on erectile tissues, while GH therapy requires time for downstream IGF-1 production to increase. However, GH therapy might offer broader systemic benefits through multiple mechanisms beyond just increasing IGF-1.
How does IGF-1 compare with other growth factors in treating ED?
Several growth factors have shown promise in treating erectile dysfunction, each with distinct mechanisms and potential advantages:
-
Vascular Endothelial Growth Factor (VEGF): Primarily promotes angiogenesis and endothelial regeneration, making it particularly valuable for vasculogenic ED. However, it has less direct impact on neuronal health compared to IGF-1.
-
Brain-Derived Neurotrophic Factor (BDNF): Excellent for neuronal regeneration following injury (e.g., post-prostatectomy ED), but with limited effects on vascular and smooth muscle components.
-
Fibroblast Growth Factor (FGF): Potent angiogenic factor that also supports smooth muscle growth, but may potentially increase fibrosis risk with long-term use.
-
IGF-1: Offers perhaps the most comprehensive approach by simultaneously addressing endothelial function, smooth muscle maintenance, and neuronal health. Its multifaceted mechanisms may make it particularly valuable for complex or multifactorial ED cases.
Combination approaches utilizing multiple growth factors may ultimately provide the most robust therapeutic responses, particularly in severe or treatment-resistant cases. Early research suggests synergistic effects when IGF-1 is combined with VEGF or BDNF in animal models of ED.
Which conditions commonly cause IGF-1 deficiency related to ED?
Several health conditions can lead to IGF-1 deficiency that may contribute to erectile dysfunction:
-
Growth Hormone Deficiency: Whether congenital or acquired (through pituitary damage, radiation, or surgery), GH deficiency leads to reduced IGF-1 production. Adult-onset GH deficiency is associated with significantly higher rates of ED, which often improves with appropriate hormone replacement.
-
Type 2 Diabetes: While total IGF-1 levels may be normal, diabetes causes "IGF-1 resistance" where cellular responses to the hormone are blunted. Additionally, glycation of IGF binding proteins alters free IGF-1 bioavailability.
-
Chronic Kidney Disease: Impairs both production of IGF-1 and tissue responsiveness, contributing to the high prevalence of ED in men with renal insufficiency.
-
Chronic Liver Disease: Reduces hepatic production of IGF-1 in response to GH stimulation, potentially contributing to the sexual dysfunction common in cirrhosis.
-
Malnutrition and Eating Disorders: Severe caloric or protein restriction significantly reduces IGF-1 production, which may contribute to ED in men with eating disorders or malabsorption syndromes.
Identifying these underlying conditions may guide treatment approaches for ED, potentially addressing the root cause rather than simply managing symptoms. In some cases, treating the primary condition (e.g., optimizing diabetes management) may improve both IGF-1 status and erectile function without requiring specific IGF-1-targeted therapies.
References
-
Feldman, H. A., Goldstein, I., Hatzichristou, D. G., Krane, R. J., & McKinlay, J. B. (1994). Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. The Journal of Urology, 151(1), 54-61.
-
Açıkgöz, A., Gökkaya, C. S., Koç, A., Tuncay, S., Aktöz, T., Nurten, A., & Nurten, R. (2016). Relationship of serum insulin-like growth factor-1 level with erectile function in patients with idiopathic erectile dysfunction. Journal of Endocrinological Investigation, 39(4), 451-455.
-
Shindel, A. W., Kishore, S., & Lue, T. F. (2016). Molecular approaches to understand human erectile physiology. Journal of Sexual Medicine, 13(11), 1646-1659.
-
Schulster, M., Bernie, A. M., & Ramasamy, R. (2018). The role of estradiol in male reproductive function. Asian Journal of Andrology, 20(2), 115-121.
-
Imrie, H., Abbas, A., & Kearney, M. T. (2010). Insulin resistance, lipotoxicity and endothelial dysfunction. Biochimica et Biophysica Acta, 1801(3), 320-326.
-
Csiszar, A., Labinskyy, N., Perez, V., Recchia, F. A., Podlutsky, A., Mukhopadhyay, P., Losonczy, G., Pacher, P., Austad, S. N., Bartke, A., & Ungvari, Z. (2008). Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. American Journal of Physiology-Heart and Circulatory Physiology, 295(5), H1882-H1894.
-
Jiang, H., Oberbach, A., & Liu, H. (2020). The relationship between insulin-like growth factor-1 and erectile dysfunction. Asian Journal of Andrology, 22(5), 454-459.
-
Musicki, B., Bhunia, A. K., Karakashian, C., & Burnett, A. L. (2015). Insulin-like growth factor-1 activates AMPK to augment nitric oxide synthase activation in the penis. Journal of Sexual Medicine, 12(5), 1210-1222.
-
Sezen, S. F., Burnett, A. L., Snyder, S. H., & Bivalacqua, T. J. (2001). Neuronal NOS-cGMP-dependent erectile function in the penis: regulatory mechanisms and therapeutic implications. Expert Opinion on Therapeutic Targets, 5(2), 241-254.
-
Lin, G., Chen, K. C., Hsieh, P. S., Yeh, C. H., Lue, T. F., & Lin, C. S. (2006). Neurotrophic effects of vascular endothelial growth factor and neurotrophins on cultured major pelvic ganglia. BJU International, 98(5), 1130-1136.
-
Zhou, F., Li, G. Y., Gao, Z. Z., Liu, J., Liu, T., Li, W. R., Cui, W. S., Bai, G. Y., Xin, Z. C. (2013). The TGF-β1/Smad/CTGF pathway and corpus cavernosum fibrous-muscular alterations in rats with streptozotocin-induced diabetes. Journal of Andrology, 33(4), 651-659.
-
Pu, X. Y., Hu, L. Q., Wang, H. P., Luo, Y. X., & Wang, X. H. (2012). Improvement in erectile dysfunction after insulin-like growth factor-1 gene therapy in diabetic rats. Asian Journal of Andrology, 14(5), 791-796.
-
Fandel, T. M., Bella, A. J., Lin, G., Tantiwongse, K., Lin, C. S., Pohl, J., & Lue, T. F. (2009). Intracavernous growth differentiation factor-5 therapy enhances the recovery of erectile function in a rat model of cavernous nerve injury. Journal of Sexual Medicine, 6(6), 1866-1875.
-
Hinkel, R., Trenkwalder, T., & Kupatt, C. (2011). Gene therapy for ischemic heart disease. Expert Opinion on Biological Therapy, 11(6), 723-737.
-
Juul, A., Bang, P., Hertel, N. T., Main, K., Dalgaard, P., Jørgensen, K., Müller, J., Hall, K., & Skakkebaek, N. E. (1997). Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. The Journal of Clinical Endocrinology & Metabolism, 78(3), 744-752.
-
Friedrich, N., Thuesen, B., Jørgensen, T., Juul, A., Spielhagen, C., Wallaschofski, H., & Linneberg, A. (2012). The association between IGF-I and insulin resistance: a general population study in Danish adults. Diabetes Care, 35(4), 768-773.
-
Pu, X. Y., Wang, X. H., Gao, W. C., Yang, Z. H., Li, S. L., Wang, H. P., & Wu, J. B. (2012). Insulin-like growth factor-1 restores erectile function in aged rats: modulation the integrity of smooth muscle and nitric oxide-cyclic guanosine monophosphate signaling activity. Journal of Sexual Medicine, 9(6), 1559-1569.
-
Zhou, F., Xin, H., Liu, T., Li, G. Y., Gao, Z. Z., Liu, J., Li, W. R., Cui, W. S., Bai, G. Y., Park, N. C., & Xin, Z. C. (2013). Effects of icariside II on improving erectile function in rats with streptozotocin-induced diabetes. Journal of Andrology, 33(5), 832-844.
-
Chen, X., Yang, Q., Zheng, T., Bian, J., Sun, X., Shi, Y., Liang, X., Gao, G., Liu, G., Deng, C., & Zhang, Y. (2016). Neurotrophic effect of adipose tissue-derived stem cells on erectile function recovery by pigment epithelium-derived factor secretion in a rat model of cavernous nerve injury. Stem Cells International, 2016, Article ID 5161248.



Comments (0)
Back to News